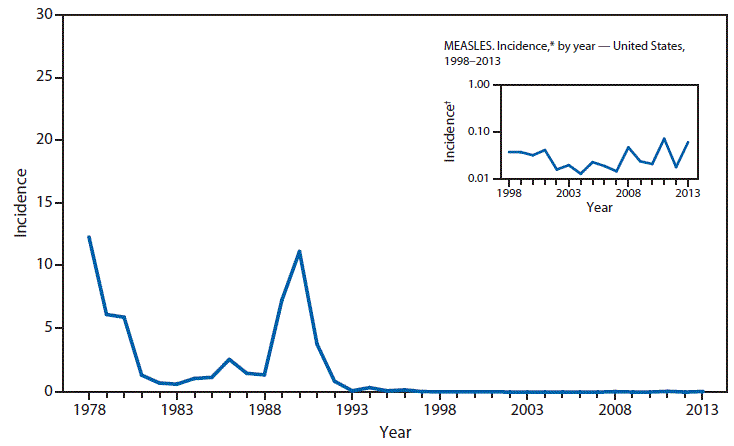
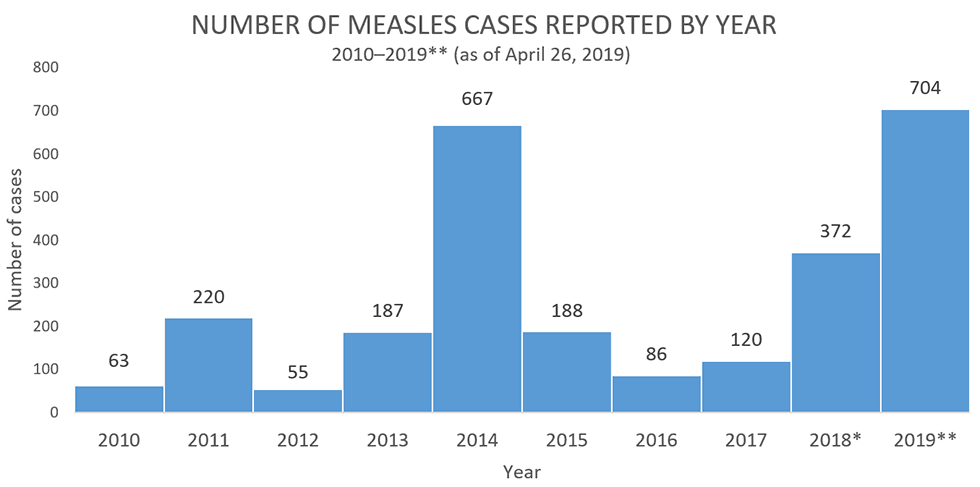
26 December 2018
Congressman
Burton Called for Recall of Thimerosal Vaccines: Eighteen years ago, after significant review
of published and unpublished scientific literature, and hearing from experts, Congressman
Dan Burton, then Chairman of the U.S. House of Representatives’ Oversight and
Government Reform Committee during the July 2000 hearing called for an
immediate recall of thimerosal containing vaccines. Several months later, he
sent a letter to then Secretary of Health Donna Shalala. In this letter, Chairman Burton wrote, “At
the time of the hearing, I requested that the Food and Drug Administration
(FDA) recall all thimerosal-containing vaccines from the market. This request
was ignored. A petition to the FDA from the parents of vaccine-injured children
was ignored. Additional scientific data that has been provided to the FDA
regarding the dangers of thimerosal in vaccines has been ignored. I am asking
that you personally respond to this request regarding an FDA recall of thimerosal
containing vaccines. During a review required by the Food and Drug
Modernization Act, it was learned that infants receive more mercury in the first
six months of life than is considered safe according to federal guidelines…
While the FDA proposes to “phase out” thimerosal-containing vaccines over time,
I implore you to conduct a full recall of these products. If the only action
that HHS takes is a gradual phase out, children will continue to be put at risk
every day. HHS is leaving supplies of this toxic substance in doctors’ offices,
at Public Health Clinics, and in managed care facilities. These vaccines will
continue to be injected in children for years to come – putting our nation’s
most vulnerable population – our babies – at risk for mercury poisoning. We all
know and accept that mercury is a neurotoxin, and yet the FDA has failed to
recall the 50 vaccines that contain thimerosal…Our children are the future of
this country. As a Government we have a responsibility to do everything within
our power to protect them from harm, including insuring that vaccines are safe
and effective. Every day that these mercury-containing vaccines remain on the
market is another day HHS is putting 8,000 children at risk. Given that
thimerosal-free vaccines are available, and the known risk of mercury toxicity,
to leave thimerosal-containing vaccines on the market is unconscionable…”(1)
Secretary
Shala Refuses: Secretary
Shalala ignored the request, and the FDA moved slowly to phase out thimerosal
in infant vaccines. Infant vaccines
containing thimerosal remained in used in the United States until at least
2003. Thimerosal continues to be used in
many flu vaccines, other adult vaccines; and thimerosal continues to be used in
vaccines globally. About the time
thimerosal was phased out of infant vaccines, a recommendation to give flu
shots to all pregnant women was initiated.
Millions of women have been and continued to be administered flu shots
that contained thimerosal. Thimerosal is also in other medical products still
used in the US as well as in cosmetics globally.
2018
– Evidence Shows He Was Correct: Congressman Burton has now retired from
Congress; in January 2019, Donna Shalala will be sworn in as a new member of
Congress; and thimerosal continues to be used in vaccines. The more I have looked at this issue and the
more I read from internal documents obtained through the Freedom of Information
Act (FOIA) process, the more I believe that Chairman Burton was right in 2000;
there was a need to recall thimerosal containing vaccines, to immediate cease
its use, and to protect our nation’s children from needless exposure to a known
neurotoxin.
Protecting
Public Health or Industry? As
those who have followed this issue know all too well, the federal agencies
involved in this issue, the US Food and Drug Administration (FDA) and the
Centers for Disease Control and Prevention (CDC), both part of the US Public
Health Service initially joined forced with the American Academy of Pediatrics
(AAP) in issuing a public statement calling for the removal of thimerosal. (2) As Congress and parents began to pay
attention, the FDA, CDC and the AAP shifted their position to be together in
lockstep to protect thimerosal’s use in vaccines. Their allegiance to protecting thimerosal rather
than children was confirmed in the stances taken during the negotiations of the
Minamata Convention on Mercury in which
the AAP joined with vaccine makers to promote an exemption for thimerosal from
the treaty. I was at the State Department for a very heated discussion on the
topic. Those who profess to protect the
public health fought in favor of keeping it in vaccines and other medicines in
2013. Lewis Carol could have had a field
day crafting this alternate reality for a those who protect a known neurotoxin
rather than babies and the planet.
Did She Perjure Herself? In the 2012 Autism hearing before the House Oversight Committee then chaired by Congressman Darrell Issa, (https://www.youtube.com/watch?v=mrEKD8zNFXI) CDC’s Congressman Dan Burton reiterated his request to get mercury out of vaccines. During this hearing, CDC’s Dr. Coleen Boyle testified, “The IOM has evaluated this issue back in 2004 and again most recently in 2011. And you know, their conclusion, again, it is not just looking at the work that was done at the CDC but with a total body of evidence was suggesting that vaccines and their components did not increase the risk of autism.“ This statement under oath contradicts the statement provided by the CDC Whistleblower in regard both to the Atlanta MMR study as well as to thimerosal as well as from the actual evidence available in research studies.
Thimerosal Use Continues Globally in 2018: That hearing was six years ago, thimerosal is still in marketplace and used in some vaccines. Neither the FDA or the CDC seem to be doing any research on thimerosal as their websites have no recent updates. I checked the National Library of Medicines, PubMed to see what had been published in the peer-reviewed literature in 2018 on the topic of thimerosal.
I found 27 articles.
What did I learn? The Total Body of Evidence on Thimerosal is Concerning. Thimerosal use may actually be increasing globally in part because of the exemption of Thimerosal in the Minamata Convention on Mercury. Some perfumes, face creams and cosmetics in India contain thimerosal. The use of cosmetics is increasing in India and as shown in the literature, globally, thimerosal is linked to allergic contact dermatitis (ACD) in between 1 and 15.5 percent of those tested. Thimerosal continues to be used in both eye and ear medications even in the United States.
I learned:
· Thimerosal allergic reactions may be delayed even as much as a week in one study; and that long exposure in an allergic dental patient took nine months for the lesions in her mouth to clear;
· ACD was reported in the US in a senior citizen using ear drops that contained thimerosal;
· A number of clinicians reporting thimerosal-linked ACD raised concerns about their colleagues not considering thimerosal during evaluations;
· Thimerosal and aluminum are present in human breast milk;
· Thimerosal free oral cholera vaccine has been developed and shows promise in helping increase the amount of cholera vaccines available globally;
· Ethylmercury (a component of thimerosal) caused abnormal neurogenic inflammatory reactions and alterations in the neuroimmune cells that remained for a longer period in the brain than in the blood;
· Exposure to thimerosal caused dysfunction that leads to impaired dopamine function and behavioral abnormalities, ultimately causing oxidative stress-related neurotoxicity;
· A neonatal dose-dependent exposure in rats to Thimerosal mimicking the childhood vaccine schedule can induce abnormal social interactions and stereotyped behaviors similar to those observed in neurodevelopmental disorders such as autism, and, for the first time, revealed that these abnormalities may be ameliorated by ALA. This indicates that ALA may protect against mercurial-induced abnormal behaviors;
· A 79-year-old woman with normal hearing developed acute bilateral sensorineural hearing loss two days after a seasonal influenza vaccination, other obvious reasons for acute hearing loss were excluded;
· A review of data from the Vaccine Safety Datalink concluded, “a dose-dependent association between increasing organic Hg exposure from Thimerosal-containing hepatitis B vaccines administered within the first six months of life and the long-term risk of the child being diagnosed with premature puberty”;
· A review of the Vaccine Adverse Events Reporting System provided, “suggestive evidence of an association between Thimerosal and neurodevelopmental outcomes and provides support for carrying out additional well-designed studies examining the association between Thimerosal-containing vaccines and a wide range of neurodevelopmental outcomes”;
· Two analyses of National Health and Nutritional Examination Survey (NHANES) data raise concerns dose dependent outcomes related to mercury, Attention Disorders, and Special Education Services Requirements;
· Several studies evaluating the effects of thimerosal exposure to both human and animal cell lines showed the toxic effect – causing cell-death for instance;
· “The comparable neurotoxicity of MeHg and EtHg has been established in vitro and in experimental animals. Studies dating back to 1985 unequivocally demonstrated that at a comparable dose, depending on the system tested, EtHg was either equal to or more neurotoxic than MeHg.”
· “Hg in combination with other neurotoxic mixtures may elevate risks of neurotoxicity, but these risks arise in circumstances that are not yet predictable. Therefore, to achieve the goals of the Minamata treaty and to safeguard the health of children, low levels of mercury exposure (in any chemical form) needs to be further reduced whether the source is environmental (air- and food-borne) or iatrogenic (pediatric TCVs):
· The NIH has funded research that is looking at numerous existing drugs including thimerosal for alternative uses; and
· In at last one California University, pharmacy students now have mandated training to develop their communication and persuasion skills to change the minds of “vaccine-hesitant” individuals. Thimerosal’s use as a preservative is among the issues included in this training.
I have made no attempt to evaluate the quality of each of these studies or pass judgement on any of the authors. To my knowledge, given the inclusion in PubMed, these are all peer-reviewed papers or review/case reports that the editors of the respective journals cleared for publication. Brazil scientists seem to be leading the way on raising concerns about the safety of thimerosal and calling for its elimination. Iranian scientists have published two papers in 2018 addressing the topic. It is important to note that on at least 3 occasions mercury treated agriculture resulted in serious injury and death among Iranians.
Dr. Boyle was incorrect in her testimony at the time. I know this because I had reviewed the ‘total body of evidence’ available at the time of her testimony and being present in the room when she gave that testimony, I have concern that she intentionally attempted to misinform the members of the Committee of the facts. Most members of the public including doctors believe ‘we got the thimerosal out of vaccines’ in 2000. Few people who are not actively engaged in this know about the Minimata Treaty, about the ongoing research worldwide that validates the risks already known in 2000, or that thimerosal is used in other medicines other than vaccines; in fragrances, face creams, and cosmetics globally.
The truth is and has always been that all forms of mercury have risks, including thimerosal. That was true in 2000 when Congressman Burton asked for a recall and its elimination; it was true in 2012, and it is true in 2018. It is past time that the FDA and the global regulatory community banned its use in any product humans are exposed to – medicines including vaccines; cosmetics, perfumes, skin creams, fungicides.
Any public health professional who touts its safety has obviously not read the literature. Those who point to the outcomes of the Institute of Medicine study is either ignorant about or has chosen to ignore the controversies and irregularities created by their contractual relationship with the CDC and the internal ‘pre-determination’ that has not been made public. Any doctor, nurse, pharmacist or other health professional who suggests that it is okay to inject pregnant women with thimerosal; to inject babies with thimerosal is violating their very ethical codes of ‘first do no harm’.
Congressman Burton evidence-based decision making in 2000 was correct. The evidence in 2018 continues to support him.
As always, the opinions expressed in this Blog are purely my own.
Beth Clay
Summary of the 27 Thimerosal Papers of 2018:
There is an entire body of articles that describe what comprises the evidence-base of science and the research hierarchy. At the top of the hierarchy are randomized controlled, double blinded placebo controlled trials. That is followed by human studies, animal studies, all the way to surveys, case reports, and epidemiology.
Human Studies
Breast Milk Study: A study conducted in Brazil was conducted to measure the total concentration of six neurotoxic elements in banked human milk. Breast feeding is universally recommended, especially in the first six months of life. Human milk is prescribed in Brazil and many parts of the world for premature and critically ill infants in neonatal units when the mother’s own milk is insufficient or not available. The study measured Hg-Mercury; Al – Aluminum, Cd – Cadmium, Pb – Lead, As-Silver, and Mn- Manganese in samples form 106 donors were obtained through the hospital-based milk bank. Thimersol containing vaccine exposure for their infants was obtained from the child’s vaccination card. The study confirmed that the “metal concentration was mostly below the limit of detection (LOD) for Cd (99%), Pb (84%), and Hg (72%), and it was above the LOD for As (53%), Mn (60%), and Al (82%), respectively. Median concentrations (dry weight) of Al, As, Hg, Mn, and Pb were 1.81 μg/g, 13.8 ng/g, 7.1 ng/g, 51.1 ng/g, and 0.43 μg/g, respectively.
Aluminum was “singly the most frequent element to which infants are exposed. Occurring binary combination (> LOD) was 56% for Al-Mn, 41% for Al-As, 22% for Al-Hg, and 13% for Al-Pb. In 100% of neonates, exposure to Al-ethylmercury (EtHg) occurred through immunization with thimerosal-containing vaccines (TCV). Association rules analysis revealed that Al was present in all of the multilevel combinations and hierarchical levels and that it showed a strong link with other neurotoxic elements (especially with Mn, As, and Hg).
(a) Nursing infants are exposed to combinations of neurotoxicants by different routes, dosages, and at different stages of development;
(b) In breastfed infants, the binary exposures to Al and total Hg can occur through breast milk and additionally through TCV (EtHg and Al);
(c) The authors concluded, “The measured neurotoxic elements were found at low frequencies in breast milk and at concentrations that pose no public health concerns for milk banking.” This study begins to scratch the surface of an issue that has raised significant concern among safety experts, that is the combined effects on exposure to aluminum and ethylmercury in utero and as newborns.(3)
Oral Cholera Vaccine Study: A randomized, observer-blinded, equivalence trial comparing the thimerosal preserved verses the thimerosal free whole cell oral cholera vaccine (Euvichol) in both adults and children in the Philippines. The objectives of this study were to assess safety and immunogenicity and to demonstrate the equivalence of the already WHO PQ formulation (100L fermenter, with thimerosal) to the scaledup formulation (600L fermenter, thimerosal-free).The study was conducted by scientists employed the manufacturer and funded by the Gates Foundation. The authors reported on 2 serious adverse events in the pediatric patients, had a vibrant discussion of active adverse event monitoring over the two month study, and discussed a lack of stratification across age groups of the GMT – “Overall, Test vaccine was immunogenic in both adults and children. The equivalence of the two Euvichol variations was confirmed on the overall analysis of combined age cohorts with a statistical power >90%. However, due to an observed geometric mean titers (GMT) Coefficient of Variation higher than expected (CV range: 1.2–6.7), the immunogenicity analysis by age cohort did not reach 90% power to demonstrate the equivalence of study agents by age strata.” The conclusion smoothed the GMT issue over, “The results of this study demonstrate the equivalence of thimerosal-free 600L Euvicho with the originally licensed Euvichol formulation (100L with thimerosal) in healthy Filipino children and adults. Based on the GMTs in the overall population, the immunogenicity of the two vaccines is equivalent for O1 Inaba and Ogawa and O139. In addition, the safety profile of the two vaccines is similar. This manufacturing of Euvichol to 600L scale may significantly contribute to the GAVI objective of expanding the current global OCV stockpile to at least 20 million doses by 2018 and also to increase the public market supply.”(4)
Allergic Contact Dermatitis Studies
Allergic contact dermatitis (ACD) is a delayed type of hypersensitivity from contact with a specific allergen to which the patients has developed a specific sensitivity.
· In the United States, a discussion article was published in Dermatitis in January reporting on the case of a female patient who presented with pruiritis and scaling of her ear canal (for several months). A series of patch tests were conducted to determine which of the agents she had been exposed to (in her medications, etc.) might be the cause. Clinically relevant positive reactions were noted for thimerosal. The article also notes that thimerosal can be found in “otic suspensions, ophthalmic solutions, nasal preparations, vaccinations, hormone injections, cosmetics, and tattoo ink.” It notes that the FDA has limited thimerosal in “dermatologic medications and cosmetics because of concerns of adverse events from mercury absorption or sensitization.” They note a positive reaction rate of 10.2% in patch testing and warn clinicians not to become complacent about thimerosal when looking for relevant exposures.(5)
· A single-center observational study conducted in Spain reports on the allergenic response to a topical use of thimerosal known in Spain as merbromin (mercurochrome in the US). “Of the 105 patients studied, 1.9% had a positive patch test to merbromin.”(6)
· In a retrospective records-based study of 58 patients in India who has potential allergic contact dermatitis (ACD) were evaluated via patch testing. A positive skin patch test for thimerosal was the second most common outcome found (15.5 % of patients). In India, thimerosal is found in face creams, eye cosmetics, and perfumes. The study noted the dramatic upsurge in the use of cosmetics. The authors called for inclusion of these comman allergens such as thimerosal to be included in the common patch testing. There was no discussion in the paper about the other known dangers of thimerosal.(7)
· The aim of this descriptive case study conducted at the University of Sarjevo in Bosnia and Herzegovina was to evaluate the results of epicutaneous patch testing with standard series of contact allergen in patients suspected to have ACD. A patch test study on 355 patients found thimerosal was the third most prevalent positive reaction found in 8.7% of patients.(8)
· A retrospective, noninterventional cohort study of 100 adolescents (aged 13-18; 74 girls, 26 boys) who were consecutively patch tested in Hungary. Contact hypersensitivity in 51 of the 100 patch-tested patients “(51%): 52.7% of the girls and 46.2% of the boys were sensitized.” The second most common allergen was thimerosal (12%). Additional findings include that reactions did not appear until the seventh day in 13% of the patients.(9)
Animal Studies
· A research study conducted in Iran: “Evidence suggests that the effect of heavy metals on neuroimmune cells lead to neurogenic inflammatory responses. In this study, immune cells [mast cells (MCs) and microglia] and pro-neuroinflammation cytokines (interleukin-1b and tumor necrosis factor-alpha) were assessed in the prefrontal lobe of rat brains exposed to thimerosal in different timeframes. A total of 108 neonatal Wistar rats were divided into three groups having three subgroups. The experimental groups received a single dose of thimerosal (300 mug/kg) postnatally at 7, 9, 11, and 15 days. The vehicle groups received similar injections of phosphate-buffered saline in a similar manner. The control groups received nothing. Samples of the prefrontal cortex were collected and prepared for stereological, immunohistochemical, and molecular studies at timeframes of 12 or 48 h (acute phase) and 8 days (subchronic phase) after the last injection. The average density of the microglia and MCs increased significantly in the experimental groups. This increase was more evident in the 48 h group. At 8 days after the last injection, there was a significant decrease in the density of the MCs compared to the 12 and 48 h groups. Alterations in pro-inflammatory cytokines were significant for all timeframes. This increase was more evident in the 48 h group after the last injection. There was a significant decrease in both neuroinflammatory cytokines at 8 days after the last injection. It was found that ethylmercury caused abnormal neurogenic inflammatory reactions and alterations in the neuroimmune cells that remained for a longer period in the brain than in the blood.”(10)
· A Team of scientists out of Brazil have just published a study in the Royal Society of Chemistry’s journal Metallomics. Noting that recent studies identified the neurotoxic effects of thimerosal (THIM), including malfunction of the monoaminergic system, the research team used the fruit fly Drosophila melanogaster to further understand the underlying cytotoxic mechanisms. “We focused on the dopaminergic system, and the rate-limiting enzyme tyrosine hydroxylase (DmTyrH), to test the hypothesis that THIM can impair dopamine (DA) homeostasis and subsequently cause dysfunction. We studied the effect of THIM by feeding 1–2-day old flies (both sexes) food supplemented with 25 μM THIM for 4 days and determined THIM-induced effects on survival, oxidative stress, and metabolic activity based on MTT assay and acetylcholinesterase (AChE) activity. Our results demonstrate that D. melanogaster exposed to THIM present changes in DmTyrH expression and activity, together with altered DA levels that led to impaired motor behavior. These phenotypes were accompanied by an increase in oxidative stress, with a decrease in MTT reduction, in AChE activity, and also in survival rate. These findings suggest an initiating and primary role for THIM-mediated DmTyrH dysfunction that leads to impaired DA function and behavioral abnormalities, ultimately causing oxidative stress-related neurotoxicity.”(11)
· Researchers investigate the impact of thimerosal containing vaccines on gut microbial succession in rhesus macaques (Primates). They note “Molecular mechanisms of thimerosal and EtHg transport within the body are not well understood. Human infants injected with thimerosal-containing vaccines (TCVs) showed detectable mercury in stool samples, which suggests that mercury potentially interacts with the gut microbiome. The authors noted, “it is not clear whether pediatric vaccines would alter the gut microbiota structure and/or function measured through the fecal metabolome.” Seeking to answer this question, the researchers investigate evaluated samples of fecal material gathered in a previous study to look at differences in microbial functionality as a consequence of vaccination were assessed. By comparing the results from macaques receiving vaccines according to the recommended 1990s and 2008 schedules with a control group (receiving only saline injections), it is possible to observe how thimerosal exposure through either pre-natal or post-natal routes could impact the gut microbiota in infant and juvenile macaques. The authors provide that “Once a TCV is administered, it is immediately dispersed in the blood stream. Thimerosal likely goes to the liver where it is broken down into EtHg and thiosalicylic acid, and possibly from EtHg to inorganic Hg through enzymatic activity. The researchers found that neither the structure nor metabolic function of the gut microbiota was significantly different between animals in the 1990s and control groups at the Infant time point. These results suggest that the single dose of thimerosal at birth from vaccination with the HepB vaccine did not have a significant impact on the gut microbiota. There is limited understanding of whether injected thimerosal or its metabolic products can be transferred through the placenta to enter the uterus. Although the placenta is impermeable to inorganic Hg, organic mercury such as methylmercury (MeHg) crosses the placenta and can accumulate within the fetus possibly disturbing fetal brain development. It is therefore conceivable that thimerosal itself, or its metabolite EtHg, could pass through the placenta, impact the fetal gastrointestinal tract, and thus impact the establishment of gut microbiota in the neonate before EtHg is further broken down into inorganic Hg within the maternal organs. However, both microbiota and metabolome analyses did not show significant differences between the group receiving the 2008 vaccine schedule (which included a prenatal influenza vaccine) and the control group at the Infant time point, suggesting that thimerosal injected via a TCV during the last month of pregnancy in rhesus macaques, does not significantly affect the neonatal gut microbiota. There were other differences noted among the animals, but not specific to the thimerosal. The author notes numerous limitations to the study ranging from the small sample size. “This is the first study to our knowledge that has measured the impact of vaccination, especially TCVs, on macaque infant gut microbial succession through metabolomic and microbiota analysis of infants soon after birth and of juveniles at 18 months of age. In this controlled animal study, the primary impact on the gut microbiome was age. We noted a few statistically significant differences on the gut microbiome structure between vaccinated and non-vaccinated groups when only animals without any medication were analyzed, but these differences were small, and appeared to be positive changes.”(12)
· A rat study conducted in Iran looked at the protective protective effects of Alpha Lipoic Acid (ALA) against thimerosal. “Thimerosal, a mercury-containing preservative has been widely used in a number of biological and drug products, including many vaccines, and has been studied as a possible etiological factor for some neurodevelopmental disabilities. Here, the protective effects of Alpha Lipoic Acid (ALA), an organosulfur compound derived from Octanoic Acid, on Thimerosal-induced behavioral abnormalities in rat were examined. 108 Wistar Rats were divided into 3 groups. “Thimerosal at different doses (30, 300, or 3000 μg Hg/kg) in four i.m. injections on 7, 9, 11, 15postnatal days. 2) ALA (at doses of 5, 10 and 20 mg/kg), following the same order; 3) single dose of Thimerosal (3000 μg Hg/kg) plus ALA at different doses (5, 10 or 20 mg/kg), by the previously described method. A saline treated control group and a ALA vehicle control (0.1% NaOH) were also included. At 5 and 8 weeks after birth, rats were evaluated with behavioral tests, to assess locomotor activity, social interactions and stereotyped behaviors, respectively. The data showed that Thimerosal at all doses (30, 300 and 3000 μg Hg/kg) significantly impacted locomotor activity. Thimerosal at doses of 300 and 3000 but not 30 μg Hg/kg impaired social and stereotyped behaviors. In contrast, ALA (at doses of 5, 10 and 20 mg/kg) did not alter behaviors by itself, at doses of 20 mg/kg, it reduced social interaction deficits induced by the highest dose of Thimerosal (3000 μg Hg/kg). Moreover, ALA, at all doses prevented the adverse effects of Thimerosal on stereotyped behaviors…The results of this preclinical study, consistent with previous studies on mice and rats, reveals that neonatal dose-dependent exposure to Thimerosal mimicking the childhood vaccine schedule can induce abnormal social interactions and stereotyped behaviors similar to those observed in neurodevelopmental disorders such as autism, and, for the first time, revealed that these abnormalities may be ameliorated by ALA. This indicates that ALA may protect against mercurial-induced abnormal behaviors.(13)
Vaccine Study in Mice: Researchers from the International Vaccine Institute in Seoul, South Korea evaluated and compared the immunogenicity of the two variations (thimerosal and thimerosal-free) of oral cholera vaccine (OCV) in mice. The mice were immunized with TM-free or TM-containing Euvichol twice at 2-week interval by intranasal or oral route. “One week after the last immunization, mice were challenged with Vibrio cholerae O1 and daily monitored to examine the protective immunity against cholera infection. In addition, serum samples were obtained from mice to measure vibriocidal activity and vaccine-specific IgG, IgM, and IgA antibodies using vibriocidal assay and enzyme-linked immunosorbent assay, respectively.” The researchers found no difference in immunogencity between the thimerosal and thimerosal-free vaccines opening the door for adoption of the TM-free cholera vaccine. They also found that the mice intranasally immunized elicited higher levels of serum antibodies than those immunized via oral route. Intranasal immunization completely protected mice against V. cholerae challenge but not oral immunization.” There authors concluded no significant difference in protection between two Euvichol variations.(14)
Case Control Studies
Care Report and Literature Review. Germany doctors report a case of deafness occurring in a temporal context of an influenza vaccination in a 79-year-old woman. A 79-year-old woman with normal hearing developed acute bilateral sensorineural hearing loss two days after a seasonal influenza vaccination, other obvious reasons for acute hearing loss were excluded. CONCLUSION: This patient appears to be the first reported case of bilateral deafness following a trivalent seasonal influenza vaccination.(15)
Dental Case Report. A 33-year-old woman sought dental assistance and presented multiple unilateral lesions. During the anamnesis, she reported having been submitted to periodontal therapy. The main complaint motivating her search for professional assistance was halitosis associated with spontaneous gingival bleeding. A patch test confirmed an allergy to thimerosal, which is a ‘compound of mercury metal’. As a result, all of her dental amalgams were removed in a single appointment. Leucoplast lesions, the result of the latent allergy response to thimerosal, persisted for several months. “The findings were compatible with Lichenoid Stomatitis. After clinical-pathological correlation, the diagnosis of idiopathic oral lichenoid lesion was finally established. Nine months after the removal of all the amalgam restorations, remission of the desquamative gingivitis and disappearance of reddish-white plaques, as well as the ulcerated surface of the oral mucosa were observed. Nevertheless, the reticular leucoplast and retro commissural region lesions persisted. The complete healing of the desquamative gingivitis, after this period, however, reinforced that it was related to the development of lichenoid lesions associated with dental amalgam, as a hypersensitive response to thimerosal. Although basic periodontal therapy may have contributed to the resolution of the DG, it was not sufficient, since the complete removal of dental amalgam restorations was necessary for the complete disappearance of the erythematous areas of the gingiva.(16)
Vaccine Safety Datalink. Studies suggest a relationship between exposure to endocrine disrupters, such as mercury (Hg), and premature puberty. Hg exposure from Thimerosal-containing hepatitis B vaccine, administered at specific intervals within the first six months of life, and the child’s long-term risk of being diagnosed with premature puberty (ICD-9 code: 259.1), was retrospectively examined, using a hypothesis-testing, longitudinal case-control design on prospectively collected data, in the Vaccine Safety Datalink (VSD). Cases diagnosed with premature puberty were significantly more likely to have received increased exposure to Hg from hepatitis B vaccines preserved with Thimerosal given in the first month after birth (odds ratio (OR) = 1.803), first two months after birth (OR = 1.768), and first six months after birth (OR = 2.0955), compared to control subjects. When the data were separated by gender, the effects remained among females but not males. Female cases, as compared to female controls, were significantly more likely in a dose-dependent manner to have received a greater exposure to Hg from hepatitis B vaccines preserved with Thimerosal, given in the first six months after birth (OR = 1.0281 per ug Hg). The results of this study show a dose-dependent association between increasing organic Hg exposure from Thimerosal-containing hepatitis B vaccines administered within the first six months of life and the long-term risk of the child being diagnosed with premature puberty.(17)
Vaccine Adverse Event Reporting System (VAERS). “Investigators postulated that early-life exposure to organic mercury (Hg) significantly increases the risk of childhood neurodevelopmental disorders (NDs). The Vaccine Adverse Event Reporting System database was utilized to conduct a hypothesis testing case-control study by evaluating 3486 total adverse event reports reported following Haemophilus influenza type b (Hib) vaccination. Exposed subjects received a Thimerosal-containing formulation (HIBTITER™, Wyeth-Lederle), while unexposed subjects received a Thimerosal-free formulation (PEDVAXHIB™, Merck). Subjects were included if they received either of these two Hib vaccine formulations between 1995 and 1999. “ Autism, Developmental Delay, Psychomotor Disorder and Neurodevelopmental disoders in general were all “significantly more likely than their respective controls to receive Thimerosal-containing Hib vaccine than Thimerosal-free Hib vaccine.” Significant effects for neurodevelopmental disorder in general were observed for males (OR = 2.52, p < 0.005), but not females when separated by gender. For the outcomes that had no biologically plausible relation to Hg exposure, the cases were no more likely than their respective controls to receive Thimerosal-containing Hib vaccine than Thimerosal-free Hib vaccine. This study provides suggestive evidence of an association between Thimerosal and neurodevelopmental outcomes and provides support for carrying out additional well-designed studies examining the association between Thimerosal-containing vaccines and a wide range of neurodevelopmental outcomes.(18)
National Health and Nutritional Examination Survey (NHANES).
· A cross section study of over 4300 kids between the ages of 13 and 19 years evaluated the hypothesis that infant Thimerosal-containing hepatitis B vaccine (T-HepB) exposure would increase the risk of an ADHD diagnosis. The evaluation used the combined 1999-2004 National Health and Nutritional Examination Survey (NHANES) and analyzed demographic, immunization, socioeconomic, and health-related variables using the SAS system. Three doses of T-HepB exposure in comparison to no exposure significantly increased the risk of an ADHD diagnosis using logistic regression (adjusted odds ratio=1.980), linear regression (adjusted beta-coefficient=0.04747), Spearman’s rank (Rho=0.04807), and 2×2 contingency table (rate ratio=1.8353) statistical modeling even when considering other covariates such as gender, race, and socioeconomic status. Current health status outcomes selected on an a priori basis to not be biologically plausibly linked to T-HepB exposure showed no relationship with T-HepB. The observed study results are biologically plausible and supported by numerous previous epidemiological studies, but because the NHANES data is collected on a cross-sectional basis, it is not possible to ascribe a direct cause-effect relationship between exposure to T-HepB and an ADHD diagnosis. During the decade from 1991 to 2001 that infants were routinely exposed to T-HepB in the United States (US), an estimated 1.3-2.5 million children were diagnosed with ADHD with excess lifetime costs estimated at US $350-$660 billion as a consequence of T-HepB. Although Thimerosal use in the HepB in the US has been discontinued, Thimerosal remains in the HepB in developing countries. Routine vaccination is an important public health tool to prevent infectious diseases, but every effort should be made to eliminate Thimerosal exposure.(19)
· A cross-sectional study of more than 1100 boys aged 7-8 years of age born between 1994 and 2007 examined the potential relationship between infant exposure to mercury from three doses of Thimerosal-containing hepatitis B vaccine and the risk of boys being adversely affected (as measured by receipt of Special Education Services – SES). Using data from the combined 2001–2014 National Health and Nutritional Examination Survey (NHANES). A robust association between three doses of infant thimerosal containing hepatitis B vaccine (T-HepB)and receipt of SES in comparison to an unexposed population was found. Covariates, such as race and socioeconomic status were taken into consideration. The authors acknowledge there are limitations, “Despite the limitation of this cross-sectional study not being able to ascribe a direct cause-and-effect relationship between exposure and outcome, it is estimated that an additional 1.2 million boys received SES with excess education costs of about United States (US) $180 billion associated with exposure to Thimerosal-containing hepatitis B vaccine. By contrast, exposure to Thimerosal-reduced hepatitis B vaccine was not associated with an increased risk of receiving SES. Therefore, routine childhood vaccination is important to reduce the morbidity and mortality of infectious diseases, but every effort should be made to eliminate Thimerosal from all vaccines.”(20)
Review Articles
Dr. Dorea from the University of Brazil published two review articles in 2018 building upon a well-respected body of evidence.
· The first stated, “All chemical forms of Hg (mercury) can affect neurodevelopment; however, low levels of organic Hg (methylmercury-MeHg and ethylmercury-EtHg in Thimerosal-containing vaccines, hereafter ‘TCV’) exposures during early life (pregnancy and lactation) co-occur with other environmental neurotoxic substances. These neurotoxicants may act in parallel, synergistically, or antagonistically to Hg. Nevertheless, the risks of neurotoxicity associated with multiple neuro-toxicants depend on type, time, combinations of exposure, and environmental and/or genetic-associated factors. Neurological developmental disorders, delays in cognition and behavioral outcomes associated with multiple exposures (which include Hg) may show transient or lasting outcomes depending on constitutional and/or environmental factors that can interact to neutralize, aggravate or attenuate these effects; often these studies are challenging to interpret.” Dr. Dorea offers, “The comparable neurotoxicity of MeHg and EtHg has been established in vitro and in experimental animals. Studies dating back to 1985 unequivocally demonstrated that at a comparable dose, depending on the system tested, EtHg was either equal to or more neurotoxic than MeHg.” This paper provides an eloquent and detailed explanation of the routes of exposure. The conclusion included: “Hg in combination with other neurotoxic mixtures may elevate risks of neurotoxicity, but these risks arise in circumstances that are not yet predictable. Therefore, to achieve the goals of the Minamata treaty and to safeguard the health of children, low levels of mercury exposure (in any chemical form) needs to be further reduced whether the source is environmental (air- and food-borne) or iatrogenic (pediatric TCVs).”(21)
· Dr. Dorea confirms that vaccines continued to be the main cause of organic mercury exposure for newborns, neonates and infants immunized with thimerosal containing vaccines (TCV) in developing countries. This paper reviews the early-life exposure to ethylmercury-EtHg and the risks associated to exposure. A review of the English language literature found, “The risk from the neurotoxic effects of pre- and post-natal Hg exposures depend, in part, on aggravating or attenuating environmental and/or genetic-associated factors.” It also noted that public health authorities (such as those at the CDC) dismiss the toxicology of mercury (immunological and subtle neurological effects as insignificant) related to low-dose Thimerosal. The review addresses the evidence that brings into question the safety of Thimerosal that is still present in vaccines given to pregnant women, infants, and children in developing countries, and recognizes the ethical imperative to extend the use of Thimerosal-free vaccines to developing countries, not just developed countries.(22)
A review article conducted at the Mayo Clinic looking at allergens in consumer products and topical medications that can cause a reaction on the skin known as allergic contact dermatitis was published in August. Thimerosal was included in the review. (23)
Use of Thimerosal for Other Purposes
A study funded by the NIH and conducted by researchers a Texas A&M “… screened 1,200 small molecules consisting of marketed drugs against C. parvum hexokinase (CpHK), from which four drugs one of which was thimerosal were identified as CpHK inhibitors with micromolar level of anti-cryptospordial activities at concentrations nontoxic to the host cells. The anti-CpHK activity of the four existing drugs provided us new reagents for studying the enzyme properties of the parasite hexokinase.(24)
Laboratory
Research of Human and Animal Cell Lines
Researchers
from four US Institutions including Thomas Jefferson University and the
University of Rochester studied Redox regulation of type-I inositol
trisphosphate receptors in intact mammalian cells.
Using
human embryonic kidney cells (HEK293) developed in 1973 from an aborted fetus
in Denmark, the researchers monitored the redox state of recombinant Ip3R1
thiols expressed in these cells when treated with thimerosal. The thimerosal
treatment modified numerous cysteines.
This is a highly technical paper which states, “To our knowledge, this
study is the first that has used proteomic methods to assess the redox state of
individual thiols in IP3R in intact cells.”(25)
Researchers
at the University of Naples using both human derived (from a four-year old
female) and rat derived cell lines, were exposed to non-toxic concentrations
(0.01 μM) of ethylmercury thiosalicylate (thimerosal) for 24 hours. “The
aim of this study was to validate the hypothesis that the exposure at non-toxic
concentrations of Thim could induce neuronal death in an in vitro model of ALS.
We found that SH-SY5Y neuroblastoma cells transfected with the G93 A mutant of
SOD1 (SOD1-G93 A) are more vulnerable to the neurotoxicant thimerosal compared to
cells overexpressing the wild type SOD1 gene (SOD1). In regard of the possible
mechanism involved in the neurotoxic effect of Thim, it should be underlined
that Thim exposure caused an increase of PDYN, a well-known DREAM target gene
and that its knocking-down by siRNA reduced the toxic effect of Thim, thus
suggesting that PDYN and DREAM can be involved, as confirmed also by the
reduction of the expression of DREAM protein in our experimental condition.
This hypothesis is reinforced by the findings showing that the knocking-down of
DREAM increased the neurotoxic effect of Thim. To our knowledge, this is the
first evidence demonstrating DREAM role in the neurotoxic effect of Thim and
its correlation with ALS physiopathology. Specifically, our results indicate
that in these experimental conditions DREAM is neuroprotective and is in
accordance with a recent paper demonstrating that in motoneurons of ALS
patients DREAM is involved in ALS physiopathology.”
Specifically,
it was reported that thimerosal, in SOD1-G93 cells, but not in SOD1 cells,
reduced cell survival. Thimerosal-induced cell death occurred in a concentration
dependent-manner and was prevented by the Sirtuin 1 (SIRT1) activator
Resveratrol (RSV). They also reported that
thimerosal decreased the protein expression of transcription factor Downstream
Regulatory Element Antagonist Modulator (DREAM), but not DREAM gene. Interestingly,
DREAM reduction was blocked by cotreatment with RSV, suggesting the
participation of SIRT1 in determining this effect. Immunoprecipitation
experiments in SOD1-G93 A cells exposed to thimerosal demonstrated that RSV
increased DREAM deacetylation and reduced its polyubiquitination. In addition,
RSV counteracted thimerosal-enhanced prodynorphin (PDYN) mRNA, a DREAM target
gene. Furthermore, cortical neurons transiently transfected with SOD1-G93 A
construct and exposed to thimerosal (0.5 μM/24 h) showed a reduction of DREAM
and an up-regulation of the prodynorphin gene. Importantly, both the treatment
with RSV or the transfection of siRNA against prodynorphin significantly
reduced thimerosal-induced neurotoxicity, while DREAM knocking-down potentiated
thimerosal reduced cell survival. These results demonstrate the particular
vulnerability of SOD1-G93 A neuronal cells to thimerosal and that RSV via SIRT1
counteracts the neurodetrimental effect of this toxicant by preventing DREAM
reduction and prodynorphin up-regulation.(26)
A
study in Brazil evaluated evaluating the interaction between bovine serum
albumin (BSA) and thimerosal (TM), an organomercury compound widely employed as
a preservative in vaccines, was investigated simulating physiological
conditions and using different spectroscopic techniques. The authors concluded, “It was proven that
both thimerosal and ethylmercury chloride accelerate the protein fibrillation
kinetics in 42 and 122%, respectively, indicating the toxicity of these
compounds in biological systems.”(27)
From the NIH: Drug screening using assays are evaluated. The National Center for Advancing Translational
Sciences (NCATS) Pharmaceutical Collection (NPC) library containing 2816 drugs
was evaluated using the in vitro co-culture assay. From the screen, 35 potent
inhibitors (IC50 ≤1 μM) were identified, followed by 15 weaker inhibitors (IC50
1–50 μM). Moreover, many known angiogenesis inhibitors were identified, such as
topotecan, docetaxel, and bortezomib. Several
potential novel angiogenesis inhibitors were also identified from this study,
including thimerosal and podofilox.
Among the inhibitors, some compounds were proved to be involved in the
hypoxia-inducible factor-1α (HIF-1α) and the nuclear factor-kappa B (NF-κB)
pathways. The co-culture model developed by using hTERT immortalized cell lines
described in this report provides a consistent and robust in vitro system for
antiangiogenic drug screening.(28)
Vaccine Education Training: Study reporting on the training of pharmacy
students to change “a patient’s mind” in regard to vaccine hesitancy at the
University of the Pacific in Stockton, California. A two-week required practicum was described
in which pharmacy students are trained in how to respond to patients and parents
who express concerns about vaccines and vaccine ingredients. Thimerosal was one of the topics. “a two-week vaccine hesitancy learning unit
was added to the required Practicum II course as a formative component that was
not part of the students’ summative grade in the course. Practicum II is a
course designed to provide hands-on learning activities in the area of
developing subjective, objective, assessment, and plan (SOAP) notes, laboratory
diagnosis, diagnostic tests, physical assessment and professional communication.
The course is divided into small discussion groups that are led by trained teaching
assistants. The goal of the learning unit was to build upon the material
learned in the APhA certificate program and provide practice in counseling
vaccine hesitant patients. The objectives of the learning unit were to enable a
student to identify common myths associated with vaccine use, identify a
patient/parent who is vaccine hesitant, apply counter strategies in
communicating with a patient/parent who is vaccine-hesitant, and apply the art of
rhetoric when communicating with a patient/parent who is vaccine-hesitant.” Using hired actors, the students used
scenario exercises to practice their communication and persuasion skills. There
were 203 students who participated in both phases of the learning unit. Only
180 students (88.6% response rate) completed both the pre- and post-attitudes surveys,
with nine items showing significant improvement. The largest reported changes were in their knowledge
about the use of thimerosal as a preservative.(29)
Sources Cited
1. Dan
Burton CO. Letter to HHS Secretary Donna Shalala calling for recall of Thimerosal
vaccines. In: Representtaives UHo, editor. House Oversight Committee Website:
US Government; 2000.
2. Centers
for Disease C, Prevention. Recommendations regarding the use of vaccines that
contain thimerosal as a preservative. MMWR Morb Mortal Wkly Rep.
1999;48(43):996-8. PubMed PMID: 10577494.
3. Bastos
WR, Vieira SM, Manzatto AG, Dorea JG, Rubira MC, de Souza VFP, et al.
Heterogeneity of Multimedia Exposures to Neurotoxic Elements (Al, As, Cd, Pb,
Mn, and Hg) in Breastfed Infants from Porto Velho, Brazil. Biol Trace Elem Res.
2018;184(1):7-15. doi: 10.1007/s12011-017-1165-1. PubMed PMID: 28967039.
4. Russo
P, Ligsay AD, Olveda R, Choi SK, Kim DR, Park JY, et al. A randomized,
observer-blinded, equivalence trial comparing two variations of Euvichol(R), a
bivalent killed whole-cell oral cholera vaccine, in healthy adults and children
in the Philippines. Vaccine. 2018;36(29):4317-24. doi:
10.1016/j.vaccine.2018.05.102. PubMed PMID: 29895500; PubMed Central PMCID:
PMCPMC6026293.
5. Aschenbeck
KA, Warshaw EM. Clinically Relevant Reactions to Thimerosal (the
“Nonallergen”) Exist! Dermatitis. 2018;29(1):44-5. doi:
10.1097/DER.0000000000000285. PubMed PMID: 28538009.
6. Balta
Cruz S, Moreno Ribera N, Estrach Panella MT. Prospective Single-Center
Observational Study of the Allergenic Potential of Mercromina Film and Other
Common Antiseptics in Patients With Contact Dermatitis. Actas Dermosifiliogr.
2018;109(1):58-62. doi: 10.1016/j.ad.2017.06.013. PubMed PMID: 28969846.
7. Garg
T, Agarwal S, Chander R, Singh A, Yadav P. Patch testing in patients with
suspected cosmetic dermatitis: A retrospective study. J Cosmet Dermatol.
2018;17(1):95-100. doi: 10.1111/jocd.12359. PubMed PMID: 28568892.
8. Kasumagic-Halilovic
E, Ovcina-Kurtovic N. Analysis of Epicutaneous Patch Test Results in Patients
with Contact Dermatitis. Med Arch. 2018;72(4):276-9. doi:
10.5455/medarh.2018.72.276-279. PubMed PMID: 30514994; PubMed Central PMCID:
PMCPMC6195022.
9. Pap
EB, Temesvari E, Nemeth I, Sardy M, Ponyai G. Contact hypersensitivity in
adolescents. Pediatr Dermatol. 2018;35(6):769-73. doi: 10.1111/pde.13609.
PubMed PMID: 30152547.
10. Afsordeh
K, Sadeghi Y, Amini A, Namvarpour Z, Abdollahifar MA, Abbaszadeh HA, et al.
Alterations of neuroimmune cell density and pro-inflammatory cytokines in
response to thimerosal in prefrontal lobe of male rats. Drug Chem Toxicol.
2018:1-11. doi: 10.1080/01480545.2018.1465949. PubMed PMID: 29745770.
11. Bianchini
MC, Gularte COA, Nogara PA, Krum BN, Gayer MC, Bridi JC, et al. Thimerosal
inhibits Drosophila melanogaster tyrosine hydroxylase (DmTyrH) leading to
changes in dopamine levels and impaired motor behavior: implications for
neurotoxicity. Metallomics. 2018. doi: 10.1039/c8mt00268a. PubMed PMID:
30516209.
12. Hasegawa
Y, Curtis B, Yutuc V, Rulien M, Morrisroe K, Watkins K, et al. Microbial
structure and function in infant and juvenile rhesus macaques are primarily
affected by age, not vaccination status. Sci Rep. 2018;8(1):15867. doi:
10.1038/s41598-018-34019-0. PubMed PMID: 30367140; PubMed Central PMCID:
PMCPMC6203732.
13. Namvarpour
Z, Nasehi M, Amini A, Zarrindast MR. Protective role of alpha-lipoic acid in
impairments of social and stereotyped behaviors induced by early postnatal
administration of thimerosal in male rat. Neurotoxicol Teratol. 2018;67:1-9.
doi: 10.1016/j.ntt.2018.02.002. PubMed PMID: 29481853.
14. Lee EY,
Lee S, Rho S, Kim JO, Choi SK, Lee YJ, et al. Immunogenicity of a bivalent
killed thimerosal-free oral cholera vaccine, Euvichol, in an animal model. Clin
Exp Vaccine Res. 2018;7(2):104-10. doi: 10.7774/cevr.2018.7.2.104. PubMed PMID:
30112349; PubMed Central PMCID: PMCPMC6082675.
15. Kolarov
C, Lobermann M, Fritzsche C, Hemmer C, Mlynski R, Reisinger EC. Bilateral
deafness two days following influenza vaccination: a case report. Hum Vaccin
Immunother. 2018:1-2. doi: 10.1080/21645515.2018.1509657. PubMed PMID:
30118641.
16. Lopes
de Oliveira LM, Batista LHC, Neto A, Silva LB, Cimoes R, Leao JC, et al. Oral
Lichenoid Lesion Manifesting as Desquamative Gingivitis: Unlikely Association?
Case Report. Open Dent J. 2018;12:679-86. doi: 10.2174/1745017901814010679.
PubMed PMID: 30369977; PubMed Central PMCID: PMCPMC6182885.
17. Geier
DA, Kern JK, Geier MR. Premature Puberty and Thimerosal-Containing Hepatitis B
Vaccination: A Case-Control Study in the Vaccine Safety Datalink. Toxics.
2018;6(4). doi: 10.3390/toxics6040067. PubMed PMID: 30445743.
18. Geier
DA, Kern JK, Homme KG, Geier MR. The risk of neurodevelopmental disorders
following Thimerosal-containing Hib vaccine in comparison to Thimerosal-free
Hib vaccine administered from 1995 to 1999 in the United States. Int J Hyg
Environ Health. 2018;221(4):677-83. doi: 10.1016/j.ijheh.2018.03.004. PubMed
PMID: 29573974.
19. Geier
DA, Kern JK, Homme KG, Geier MR. A cross-sectional study of the relationship
between infant Thimerosal-containing hepatitis B vaccine exposure and
attention-deficit/hyperactivity disorder. J Trace Elem Med Biol. 2018;46:1-9.
doi: 10.1016/j.jtemb.2017.11.001. PubMed PMID: 29413097.
20. Geier
DA, Kern JK, Homme KG, Geier MR. A Cross-Sectional Study of the Association
between Infant Hepatitis B Vaccine Exposure in Boys and the Risk of Adverse Effects
as Measured by Receipt of Special Education Services. Int J Environ Res Public
Health. 2018;15(1). doi: 10.3390/ijerph15010123. PubMed PMID: 29329213; PubMed
Central PMCID: PMCPMC5800222.
21. Dorea
JG. Multiple low-level exposures: Hg interactions with co-occurring neurotoxic
substances in early life. Biochim Biophys Acta Gen Subj. 2018. doi:
10.1016/j.bbagen.2018.10.015. PubMed PMID: 30385391.
22. Dorea
JG. Low-dose Thimerosal (ethyl-mercury) is still used in infants` vaccines:
Should we be concerned with this form of exposure? J Trace Elem Med Biol.
2018;49:134-9. doi: 10.1016/j.jtemb.2018.05.010. PubMed PMID: 29895363.
23. Nguyen
HL, Yiannias JA. Contact Dermatitis to Medications and Skin Products. Clin Rev
Allergy Immunol. 2018. doi: 10.1007/s12016-018-8705-0. PubMed PMID: 30145645.
24. Eltahan
R, Guo F, Zhang H, Zhu G. The Action of the Hexokinase Inhibitor
2-deoxy-d-glucose on Cryptosporidium parvum and the Discovery of Activities
against the Parasite Hexokinase from Marketed Drugs. J Eukaryot Microbiol.
2018. doi: 10.1111/jeu.12690. PubMed PMID: 30222231.
25. Joseph
SK, Young MP, Alzayady K, Yule DI, Ali M, Booth DM, et al. Redox regulation of
type-I inositol trisphosphate receptors in intact mammalian cells. J Biol Chem.
2018;293(45):17464-76. doi: 10.1074/jbc.RA118.005624. PubMed PMID: 30228182;
PubMed Central PMCID: PMCPMC6231128.
26. Laudati
G, Mascolo L, Guida N, Sirabella R, Pizzorusso V, Bruzzaniti S, et al.
Resveratrol treatment reduces the vulnerability of SH-SY5Y cells and cortical
neurons overexpressing SOD1-G93A to Thimerosal toxicity through
SIRT1/DREAM/PDYN pathway. Neurotoxicology. 2018;71:6-15. doi:
10.1016/j.neuro.2018.11.009. PubMed PMID: 30503815.
27. Santos
JCN, da Silva IM, Braga TC, de Fatima A, Figueiredo IM, Santos JCC. Thimerosal
changes protein conformation and increase the rate of fibrillation in
physiological conditions: Spectroscopic studies using bovine serum albumin
(BSA). Int J Biol Macromol. 2018;113:1032-40. doi:
10.1016/j.ijbiomac.2018.02.116. PubMed PMID: 29476861.
28. Li S,
Hsu CW, Sakamuru S, Zou C, Huang R, Xia M. Identification of Angiogenesis
Inhibitors Using a Co-culture Cell Model in a High-Content and High-Throughput
Screening Platform. SLAS Technol. 2018;23(3):217-25. doi:
10.1177/2472630317729792. PubMed PMID: 28922619; PubMed Central PMCID:
PMCPMC6032403.
29. Vyas D,
Galal SM, Rogan EL, Boyce EG. Training Students to Address Vaccine Hesitancy
and/or Refusal. Am J Pharm Educ. 2018;82(8):6338. doi: 10.5688/ajpe6338. PubMed
PMID: 30425397; PubMed Central PMCID: PMCPMC6221531.