4 August 2017
On 1 August 2017, I posted a survey on Facebook to gather information about the interaction between families and their medical professionals when a suspected vaccine adverse reaction occurred. The purpose of this inquiry is two-fold:
- To gain insights into the interactions between health professionals and families when a suspected adverse reaction has taken place; and
- To gain more knowledge about how often suspected adverse reactions are reported to the US Food and Drug Administration’s (FDA’s) MedWatch or VAERS (Vaccine Adverse Events Reporting System); and
This survey was not intended to suggest that every individual receiving a vaccine experiences an adverse reaction. In fact, I am thrilled when someone does not experience a life altering adverse reaction after administration of a vaccine because I have come to know so many families whose lives have been irreparably harmed when a loved one experienced a serious adverse reaction to one or more vaccines and suffered seizures, brain swelling, or death. Vaccines, like all drugs, have inherent risks. In looking at recommendations of vaccines and other drugs, there is always supposed to be the consideration of the balance of risk versus benefit. Once a product enters the marketplace, the post marketing surveillance is key looking for unexpected adverse events as well as monitoring the number of known risks so that the risk/benefit ratio may be updated as more information becomes known.
The decision to vaccinate, like all medical decisions, is one that is best made by the individual and for children, best made by their parents. Information is key. It is one of the reasons when I am asked, that I advise individuals to go to the FDA website and read the package inserts for the vaccines they are considering in addition to any other research they are doing. The information in the package insert covers the use, contraindications, and warnings. It is the information that the FDA has reviewed with the company and required to be included. Any questions that arise from reviewing this information should be resolved before administration.
One’s own medical history and family medical history is important in making these decisions. A family history of auto-immune disorders is a red flag. A history of drug allergies or allergies to eggs, neomycin, and other vaccine ingredients is also important. Religious considerations regarding porcine, bovine, and aborted human fetal tissue being utilized in some vaccines is also important for some.
Because vaccines recommended for administration to children in the United States, including the adult versions, are different than other drugs and vaccines in that the manufacturers and those who administer the vaccines are protected from being sued by those who experience life altering medical injuries, greater focus on monitoring adverse events, and improving safety is vital.
Adverse Event Reporting
Reporting adverse reactions, even those known to occur with a drug or vaccine are important in post marketing surveillance. Government and media alike frequently report that vaccine reactions are rare and typically mild reactions. In 1999 while leading the House Oversight investigation looking at vaccine injuries, I learned that like most drugs, vaccine adverse reactions are vastly under reported. I also heard from thousands of families that they were not truly informed what reactions to look for, or what to do in the event of a serious reaction (such as spiking a high fever, or high-pitched screaming that goes on for hours – signaling a potential encephalopathy). Parents also often reported that the pediatrician would ignore the cause and effect of vaccination and subsequent sudden change in health status.
At the time of the passage of the National Childhood Vaccine Injury Act of 1986 (42 U.S.C. §§ 300aa-1 to 300aa-34), medical professionals submitted adverse vaccine reactions to the FDA in what would become known as the MedWatch system. Few studies have been done to confirm how often reports are made, since the system is a passive voluntary system. From their own data, FDA has previously suggested that only between 1 and 5.6% of drug adverse reactions are reported.
In 1990, the Vaccine Adverse Events Reporting System (VAERS) system was created as a joint activity between the FDA and the Centers for Disease Control and Prevention (CDC). https://vaers.hhs.gov/index.html
It is considered the national ‘early warning system’ for post marketing surveillance of US vaccines. Like the MedWatch system, it is a ‘passive system’. The public may make their own reports, however; the same law that created the National Vaccine Injury Compensation Program (VICP) mandated that health care professionals (physicians, etc) report vaccine adverse reactions. Vaccine manufacturers are also required to report all adverse events that are reported to them. While the mandate to report exists, the law did not include any consequence for doctors who fail to report. Learning through this survey the frequency of reports will provide evidence as to whether the system is working as Congress intended or not.
According to their website, the primary objectives of VAERS are:
- Detect new, unusual, or rare vaccine adverse events;
- Monitor increases in known adverse events;
- Identify potential patient risk factors for particular types of adverse events;
- Assess the safety of newly licensed vaccines;
- Determine and address possible reporting clusters (e.g., suspected localized [temporally or geographically] or product-/batch-/lot-specific adverse event reporting);
- Recognize persistent safe-use problems and administration errors;
- Provide a national safety monitoring system that extends to the entire general population for response to public health emergencies, such as a large-scale pandemic influenza vaccination program. ( https://vaers.hhs.gov/about.html)
About the Survey
I posed the following five questions:
- Have you or a member of your immediate family (child, spouse, parent) ever suffered a suspected adverse reaction to a vaccine?
- After the suspected vaccine reaction, did you call your doctor’s office, go to an emergency room, or seek medical input in any way?
- In looking back, did the health professionals you interacted with document and report to the FDA the suspected vaccine adverse event? (FDA manages the MEDWatch system and the VAERS system, both of which are avenues to report adverse reactions.)
- Please describe briefly the vaccine adverse event including age of individual, type of reaction, and how the medical professionals involved responded.
- For demographic purposes only, please list the state you lived in at the time (or country if outside of the USA).
No names, emails or other personally identifying information was collected from responders. While this increased the risk of trolling, I felt it important to keep the survey as short as possible and to allow responders to maintain their privacy.
In the first 72 hours the survey was open, 256 individuals responded. This resulted from two postings on Facebook. In preparing this survey initially, I did not configure the first question to go to the end if the answer was no. After reviewing these initial responses, I have since corrected that. The 72 ‘No’ responses to Question 1were excluded from further evaluation.
Responses From 184 Individuals
Question 2 was intended to gain a greater understanding of how families interacted with health professionals while dealing with your own or a loved ones suspected adverse reaction.

Question 3 was intended to gather information about whether or not the adverse event was reported.

Question 4: The responses to Question 4: “Please describe briefly the vaccine adverse event including age of individual, type of reaction, and how the medical professionals involved responded.” uncovered that 30 of the 184 responders were not serious responders but trolls – providing flippant answers.
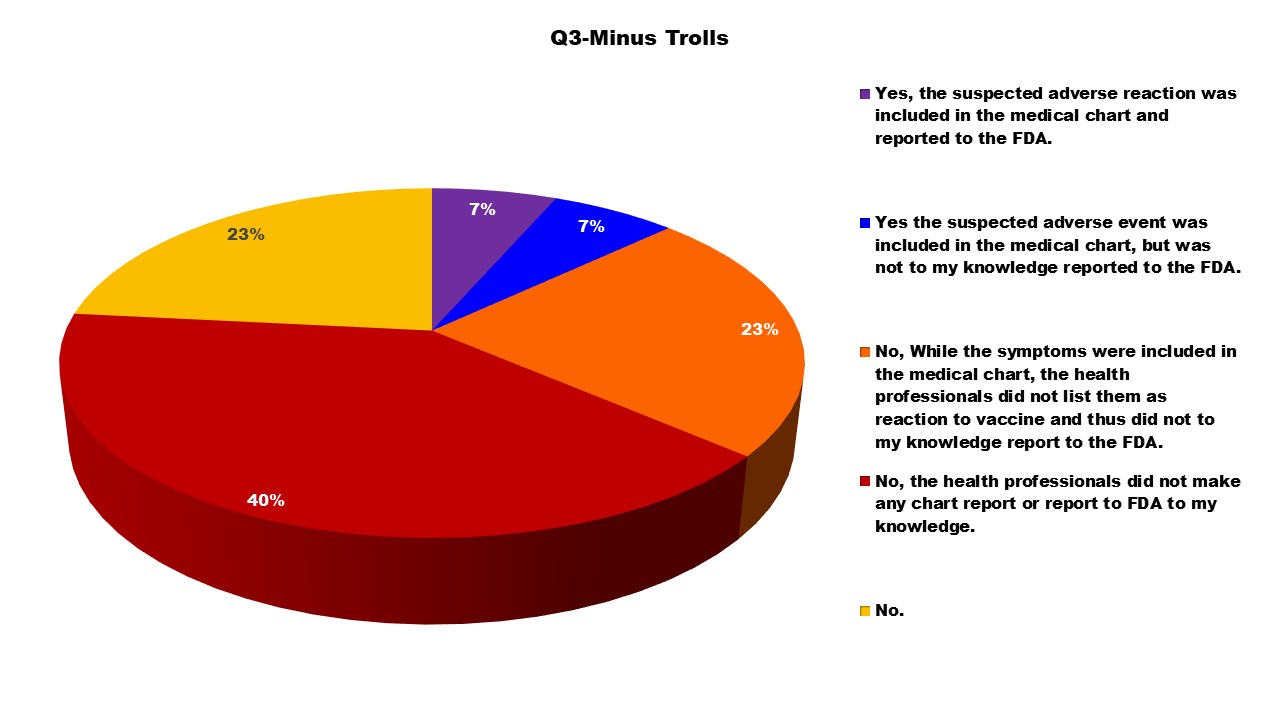
After removing the 30 trolls, the following outcomes on the Questions 2 and 3 show slightly different outcomes. The largest difference being in Q2 when initially there were more individuals who reported ‘No’.
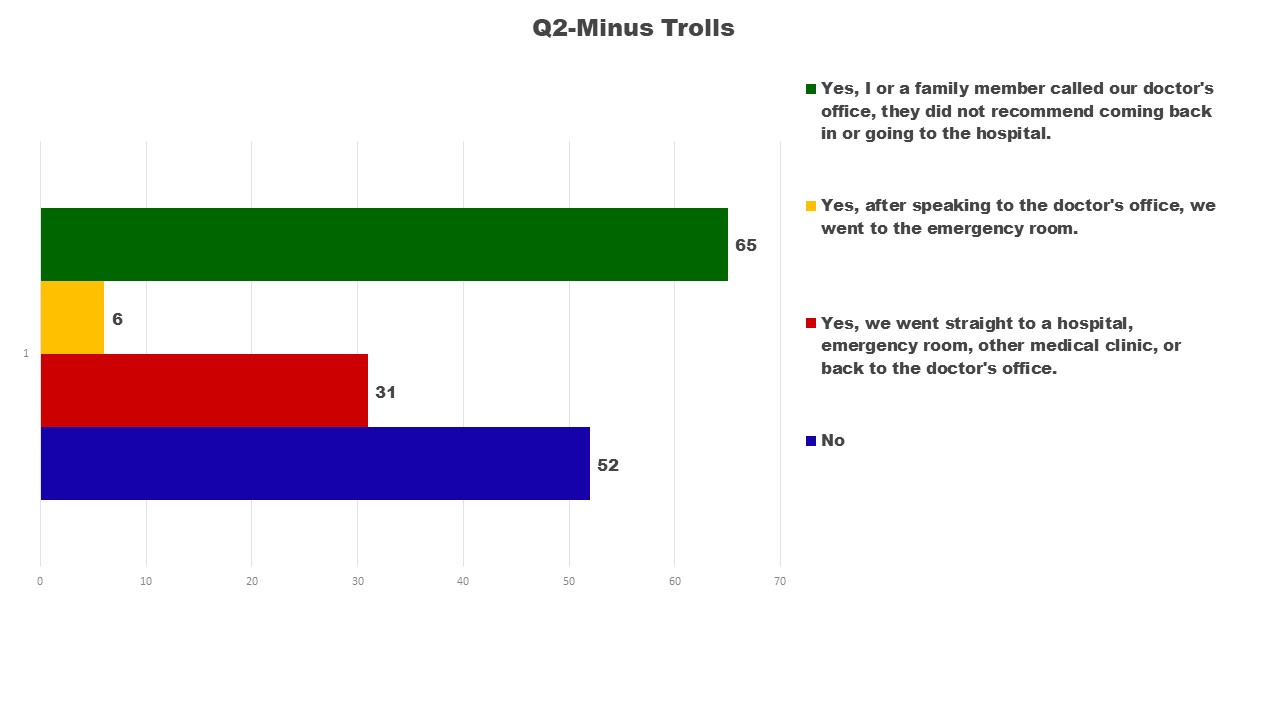
I was initially surprised by how many did not call their doctor or go to the emergency room. Some of those who did not seek medical input were dealing with mild reactions; others did not connect their child’s sudden changes to the vaccine until later. Of greater concern 59 out of 65 calls to the doctor did not result in a suggestion to come back in or go to the emergency room. If the doctor had liability for the reaction the same as other medications prescribed, would the response to these calls be any different? It is a question I cannot answer, but believe needs serious review. Are doctors trained to understand vaccine adverse reactions, or have they been discouraged from connecting the dots between vaccine administration and the onset of high fevers, brain swelling, seizures, and other adverse reactions other than anaphylactic shock. This is another issue I do not have answers for, but believe needs serious consideration.
As noted on the VAERS website, even known adverse reactions are supposed to be reported. This survey confirms that too often this is not happening. Numerous parents reported in the survey that the reactions their children were experiencing were ‘normal’ and did not need to be reported to VAERS. Only seven percent of these responders stated the adverse reaction was included in the medical chart and reported to VAERS. Ninety-three percent of adverse events were likely not reported to VAERS. Clearly there needs to be improved training to the health professions about the importance of reporting even the ‘expected’ side effects to VAERS so that FDA and CDC can better track reactions to include in their risk/benefit ratio analysis.
Question 4: Please describe briefly the vaccine adverse event including age of the individual, type of reaction, and how the medical professionals involved responded.
Many individuals did not include the age of the individual. The responses that were included ranged from the day of birth to individuals over 60. Most appeared to be parents reporting on the pediatric event. There were responses from more than 25 states, Guam (a US Territory) and several countries including Australia, Sweden, Northern Ireland, Israel, and England.
Several themes seemed to be present in the responses:
Pediatric Reactions
- High fever – 105 F and above
- Rash
- Development of gastric issues that had not been present before.
- Loss of verbal and social skills
- Head banging
- High pitched prolonged inconsolable crying or arched back screaming (one responder called it the ‘encephalitic cry’)
- Seizures
- Skin issues including eczema developed
- Autism behaviors developed
Several parents mentioned their child had the MTHFR genetic mutation. Two pediatric deaths were reported. One was attributed to a vaccine adverse reaction; the other physicians did not make the connection.
There were fewer adult responses. However, the below reactions were mentioned.
- Migraines
- Fatigue
- Abdominal pain
- Guillain Barré syndrome
- Shoulder pain
- Developed shingles from shingles vaccine
- Development of auto-immune conditions
Many parents reported that the doctor or nurse they spoke with made one of the following comments to them.
- High fever its normal, give them Tylenol or Motrin.
- The reaction is normal, no need to report it to VAERS.
Survey responders also reported frequently that the doctor or nurse was dismissive of their concerns about a reaction to a vaccine. One mother reported her two-month-old infant developed a golf ball sized lump in thigh were injected that was still present at the four-month office visit. The nurse had no concern about the swelling and was prepared to inject another vaccine into the swollen area. Mom declined vaccines on that visit.
Summary
The VAERS system appears underutilized by health professionals. Because health professionals are not fully utilizing the VAERS system, the FDA and CDC are hamstrung in their ongoing post-marketing surveillance of vaccines. This creates a domino effect on monitoring the risk/benefit ratio and undermines the mission of both agencies in relation to vaccine safety matters.
The VAERS team should increase their social media profile and capitalize on the agency’s relationships the professional medical trade associations to remind doctors, nurses, and other health professionals of the primary objects of the VAERS program:
- Detect new, unusual, or rare vaccine adverse events;
- Monitor increases in known adverse events;
- Identify potential patient risk factors for particular types of adverse events;
- Assess the safety of newly licensed vaccines;
- Determine and address possible reporting clusters (e.g., suspected localized [temporally or geographically] or product-/batch-/lot-specific adverse event reporting);
- Recognize persistent safe-use problems and administration errors;
- Provide a national safety monitoring system that extends to the entire general population for response to public health emergencies, such as a large-scale pandemic influenza vaccination program.
There is an urgent need to improve the education and awareness among health professionals of the signs and symptoms of vaccine adverse reactions including the link between high fever, inconsolable and arched back crying and possible development of encephalitis after vaccination.
Not everyone will suffer a serious reaction to a vaccine. Several respondents talked about mild reactions, but appreciate that their child was protected against the infectious diseases from their vaccines.
Vaccines like every medication can and do cause serious adverse reactions including death. Too little accurate information is known about how often a serious reaction develops and who is most vulnerable. Generals in the military develop guidance on the levels of acceptable collateral damage in the development of military maneuvers. It may seem callous, but it is one of the ways of measuring progress. Has the public health community developed their own guidance on acceptable collateral damage? If they have, they have not made it public. Is a 1% serious reaction acceptable? Would a 10% serious reaction be acceptable? If 1 in 10,000 infants die from a mandated vaccine, is that acceptable? If 1 in 1,000 develop a seizure disorder and suffer irreparable brain injury, is that acceptable? If anyone has research in which the actual percentages of vaccine reactions are included, I hope you will share it with me. Too often we have the numerator without the denominator. (vaccine reactions are the numerator, vaccines administered are the denominator).
Unless and until we have an honest dialogue free of name calling and bullying behavior and honest transparent research, we will not see the development of safer vaccines.
The survey will remain open at least through August. I will provide an update if there are significantly more responses to it in September. To take the survey, click here: https://www.surveymonkey.com/r/LZFXKJZ
Working together we can improve our knowledge base.
Always,
Beth
